The majority of Medical Devices incorporate metallic materials (Titanium, CrCo, Stainless steel, etc.). When brought close to the MRI, these devices are likely to be attracted to the intense magnetic field of the MRI.
The risk linked to this displacement force must be assessed as part of a safety study between the interactions of MRI and a Medical Device. In most cases, this evaluation consists of carrying out a test according to the ASTM F2052 standard, considering the worst possible scenario.
How to determine the worst case in ASTM F2052 testing?
The displacement force induced by the static magnetic field of MRI depends on the magnetic susceptibility of the materials and the volume of material constituting the device. Determining a worst case in a range of devices is therefore generally simple because it consists of searching for the device or the assembly which will maximizes both the volume and the magnetic susceptibility of the material.
This is slightly more complex when multiple metallic materials with different magnetic susceptibilities are involved.
Once the worst-case is identified …
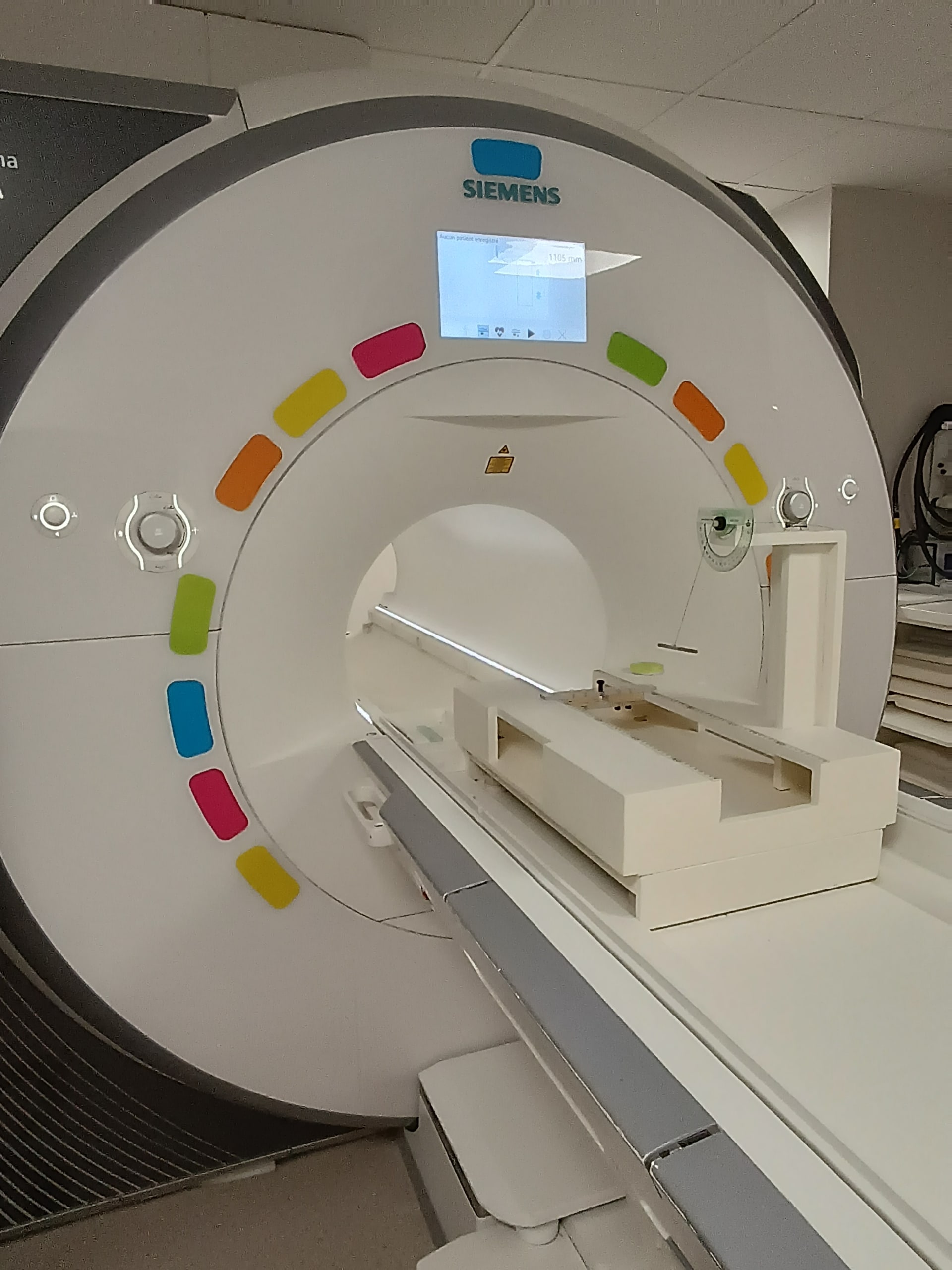
Following the determination of the worst case, a test or tests are carried out. The ASTM F2052-21 standard describes a method suitable for many devices, which consists of suspending the device, bringing it close to the MRI scanner bore centre and measuring the angle of deviation. This angle allows for the calculation of the force induced on the device under the test conditions.
The results are then interpreted based on these measurements and the maximum acceptable force.
How Healtis can help you?
We offer the following services:
- To support you in determining the worst case
- to carry out tests in compliance with ASTM F2052 and under ISO17025* accreditation
- to interpret the results of these tests to feed into the labeling of the device.
*Cofrac Testing Accreditation, N° 1-6320, scope available on www.cofrac.fr