Magnetically induced Torque in MRI : details and assessment methods
What causes magnetically induced torque in MRI?
When exposed to the main magnetic field of MRI, medical devices containing or composed entirely of magnetic materials will experience a magnetic torque.
The device tends to orient itself according to the magnetic field, in the same way a compass needle orientates itself according to the earth’s magnetic field.
This torque will depend on:
– the magnetic properties of the device,
– the volume and shape of the device,
– the intensity of the magnetic field.
A few years ago, we illustrated this phenomenon using a simple steel paperclip inserted in an eraser.
Magnetically induced torque can pose a risk to patients who have implanted medical devices, or to anyone handling a magnetic objects in the MRI environment.
ASTM F2213-17 offers 5 distinct methods for assessing the torque on medical devices.
How to assess Torque in MRI according to ASTM F2213-17?
The “Low Friction Surface Method”
The Low Friction Surface method is a qualitative, and thus the simplest, method for assessing the magnetically induced torque on a device.
By observing a lack of alignment to the main field, the low friction surface method can be used to assess an upper limit (based on the frictional force of the device) of torque and is only applicable when the expected torque is very low (ie. for devices made of materials with very low magnetic susceptibility).
Introduction of the “Low Friction Surface” method
The test procedure is as follows:
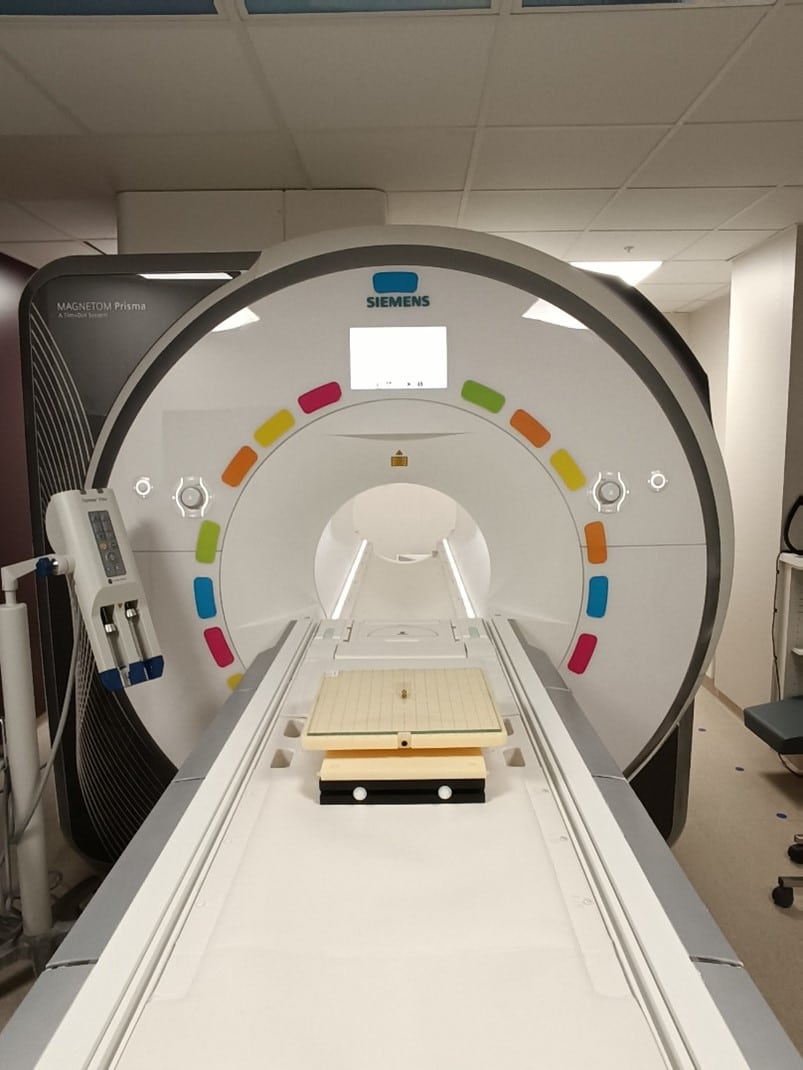
the device is placed in the center of the MRI scanner, on a low-friction surface glass plate.
The plate is rotated, in 45° increments, a full 360°.
During the rotation we observe whether the device moves under the effect of the magnetic field.
What conclusions can we draw?
If no movement is observed, we can determine an upper limit for the magnetic torque by evaluating the frictional force between the plate and the device, since the frictional force must be greater than any possible induced torque.
This upper boundary limit is then compared with the acceptance criterion.
If the device does move (eg. aligns with the main magnetic field), then we cannot draw a conclusion beyond that it experienced magnetically induced torque, and it must be subjected to a more robust magnetic torque assessment which can quantify the amount of torque, such as the “Torsional Spring” method or the “Pulley” method. (#backlink paragraphe Pulley)
This torque assessment method is included in Healtis’ ISO17025(1) scope of accreditation.
(1)HEALTIS is NF EN ISO/CEI 17025 accredited (Accréditation Cofrac Essais, N° 1-6320, scope available on www.cofrac.fr).
The “Torsional spring” Method
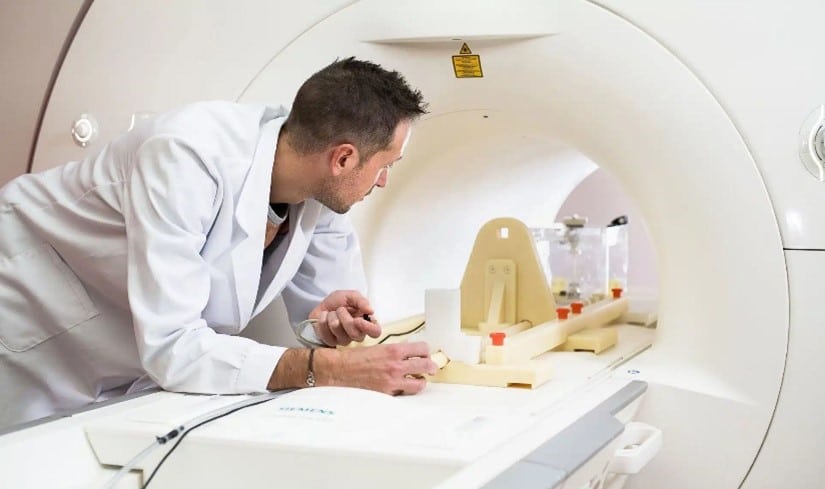
The method of assessing torque on medical devices pictured above is the “Torsional Spring” method. It is a quantitative assessment of the magnetically induced torque that utilizes a specialized test apparatus.
The test procedure is as follows:
- the device is mounted on the platform of the torsional spring test apparatus;
- the test apparatus is positioned at the center of the MRI scanner;
- the torque is measured based on the readout from the torsion spring apparatus.
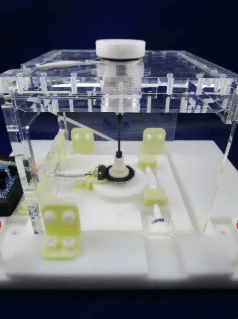
Introducing the “Torsional spring” method
The principle of the measurement is based on the properties of a torsionally-characterized rod within the apparatus that connects the top platform to a measurement unit at the base of the apparatus. When the device is placed in a static magnetic field, it tends to align itself with the magnetic field. This has the effect of twisting the rod, which acts like a spring. Based on a known spring coefficient of the rod and the angular difference between the top and bottom of the rod the torque can be measured.
Measurements are multiplied by changing the setpoint angle to measure the maximum torque.
What conclusions can we draw from the Torsional Spring method?
This method provides an accurate measure of the maximum torque that the device will undergo within the MRI.
This torque can then be compared with an acceptance criterion to determine whether there is a risk to the patient.
This torque assessment method is included in Healtis’ ISO17025(1) scope of accreditation.
(1)HEALTIS is NF EN ISO/CEI 17025 accredited (Accréditation Cofrac Essais, N° 1-6320, scope available on www.cofrac.fr).
Pulley” test method for torque evaluation according to ASTM F2213
Healtis has developed a test bench based on the “Torque Pulley” test method, one of the quantitative methods described in ASTM F2213-17.
Justine Noel, Measurement and Test Engineer at Healtis, was actively involved in the design and implementation of this test method.
She provides some details below:
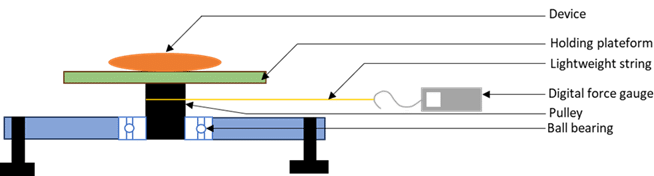
“The Pulley method measures magnetically induced torque by measuring the force required to turn the device on a pulley.
The torque (τmag ) is equal to the measured force (F) minus the friction force (Ff) multiplied by the pulley radius (r): τmag =r(F-Ff).
The friction force is the force required to complete one revolution without the test object.
This method is particularly useful for assessing the torque induced on medical devices where high torque is expected.”
Does the measured torque pose a risk?
To determine whether or not the torque poses a risk, it must be compared to an acceptance criterion.
The ASTM F2213-17 standard suggests an acceptance criterion equivalent to the gravity torque of the device. The standard proposes that the device presents no risk of potential injury from magnetically induced torque if the torque induced during MRI is lower than the torque due to the effect of gravity.
This criterion is conservative, and it is possible to define a higher acceptance criterion, for example by looking at the force required to move the device.
In summary,
Magnetically induced torque is a risk that should be assessed for any medical device that contains magnetic materials that may enter the MRI environment. While there are several methods to assess this risk,
- The “Low Friction Surface” method is a simple and effective method when the expected torque is low (1);
- The “Torsional Spring” method provides a robust assessment of maximum torque for devices which may have more significant torque (1).
- The “Pulley” method, for precise assessment of induced torque, particularly for medical devices where the expected torque is likely to be significant.
Determining which Torque method is best for the assessment of your device is not always straightforward; so, we invite you to consult with us to discuss further.
If you wish to learn more about this topic, feel free to reach out now at [email protected] !