🏁🏎 “It’s lights out and away we go”🏎 🏁
🏁🏎 “It’s lights out and away we go”🏎 🏁 We had an amazing time with the entire Healtis team during our company event at Oberlin karting in Nancy! Smiles, camaraderie, and adrenaline were all in abundance. A big thank you to all our colleagues for their participation and enthusiasm!
ASTM F2119 has officially been updated!
🔔 ASTM F2119 HAS OFFICIALLY BEEN UPDATED 🔔 After 2 years of being ‘withdrawn without replacement’, ASTM F2119 “Standard Test Method for Evaluation of MR Image Artifacts from Passive Implants” has officially been updated. If you have previously tested your medical device for image artifacts in MRI, contact us today to see how this update […]
Dental or maxillofacial systems: what MRI assessment is required?
MRI is one of the most demanding electromagnetic environments to which a medical device can be exposed. For medical devices made of metallic, magnetic or electrically conductive materials, exposure to MRI can generate risks that need to be assessed to gain access to commercial markets. What about dental and maxillofacial implant systems? Many implants, abutments, […]
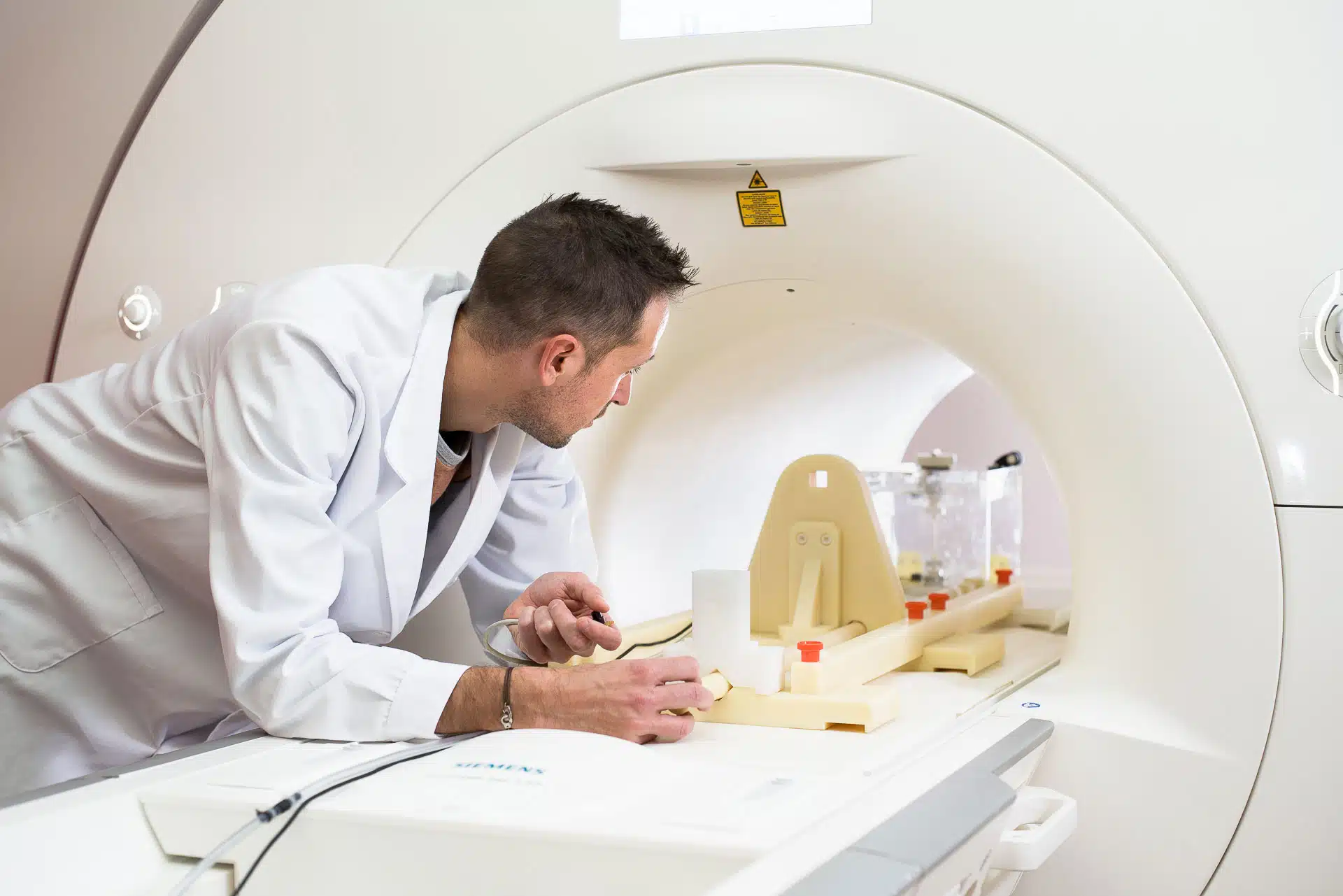
Have you ever assess the MRI compatibility of your implant range?
Have you ever assess the MRI safety of your medical devices? Should you have any questions about this matter, feel free to reach out at any time : Spencer Parent : [email protected] (USA/Canada time zones) Thibault Klammers : [email protected] (Europe)
Check out the interview with Spencer Parent, our Technical Sales Engineer at Healtis!
Our Technical Sales Engineer Spencer Parent answers a few questions about his career and his current position within the Healtis team! Read the interview below: 1/ First of all, where are you from? I was born and raised in Ottawa, Ontario – the capital of Canada. Seeing as Canada is a bilingual country, both English […]
Throwback to our Healtis event!
Throwback to our Healtis event! In November 2023, the whole team gathered at Fort Pelissier (France, 54) A few photos highlighting the activities carried out over there : strength, logic and skill games!
Assessment methods for the magnetically induced Torque in MRI
Quick overview of two assessment methods according to the ASTM F2213 : “Low Friction Surface” and “Torsional spring”.
Healtis will be present at AAOS 2024 in San Francisco!
Attention all manufacturers of Orthopaedic Implants! Healtis is excited to announce that we will be back at AAOS 2024 this year, happening in San Francisco from February 12th to 16th 2024! Spencer Parent will be present all week, discussing all things MRI Safety. Orthopaedic implants represent a large percentage of implanted medical devices and so […]
Healtis we will be present for the first time at ArabHealth!
Attention all manufacturers of medical devices! We are excited to announce that Healtis we will be present for the first time at ArabHealth in 2 weeks, happening in Dubaï (UAE)! (Jan-29 to Feb-02 2024) We are very much looking forward to discussing the importance of the MRI safety and compatibility testing of your medical devices. […]
Happy new year 2024!
Happy new year 2024! The entire Healtis team wishes you a happy and prosperous year!