MRI is one of the most demanding electromagnetic environments to which a medical device can be exposed.
For implanted medical devices made of metallic, magnetic or electrically conductive materials, exposure to MRI can generate risks that need to be assessed to gain access to key markets.
What tests must be carried out on orthopedic implant systems?
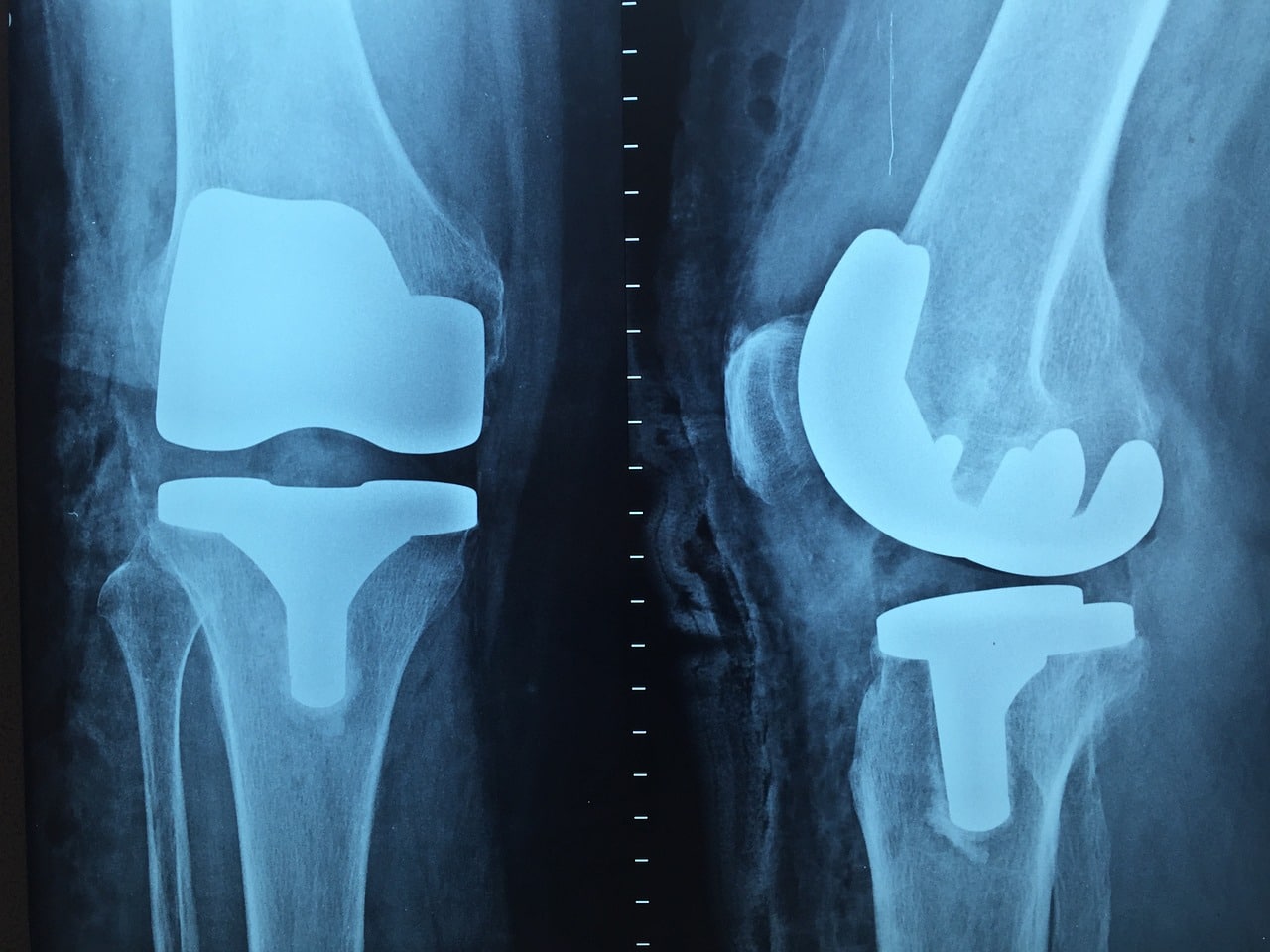
The vast majority of orthopedic systems are made of conductive materials (titanium, cobalt-chromium, stainless steel, etc.). When such a device is introduced into the MRI environment, several effects are produced, including an attractive force and torque induced by the MRI’s magnetic field, and heating due to the radiofrequencies present during the examination. Image disturbance “artifacts” are also observed in the vicinity of the device.
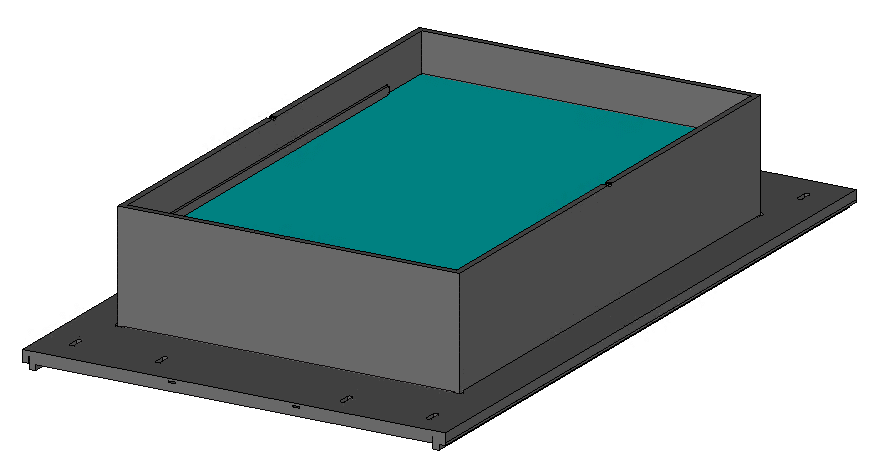
Assessing these risks generally requires testing according to ASTM standards (ASTM F2052, ASTM F2213, ASTM F2182 and ASTM F2119).
These tests are carried out on “worst-case” configurations. For magnetically induced force and torque on your device, as well as for artifacts, these worst-cases are generally easily identifiable, and a discussion with our team is usually all that’s needed to select a worst-case.
As far as radiofrequency heating is concerned, the variability of clinical configurations makes worst-case prediction complex, if not impossible, without in-depth study.
For an orthopaedic implant range, it is then necessary to search for the worstcase configuration using numerical simulations.
Focus: the use of numerical simulation to determine a worstcase configuration
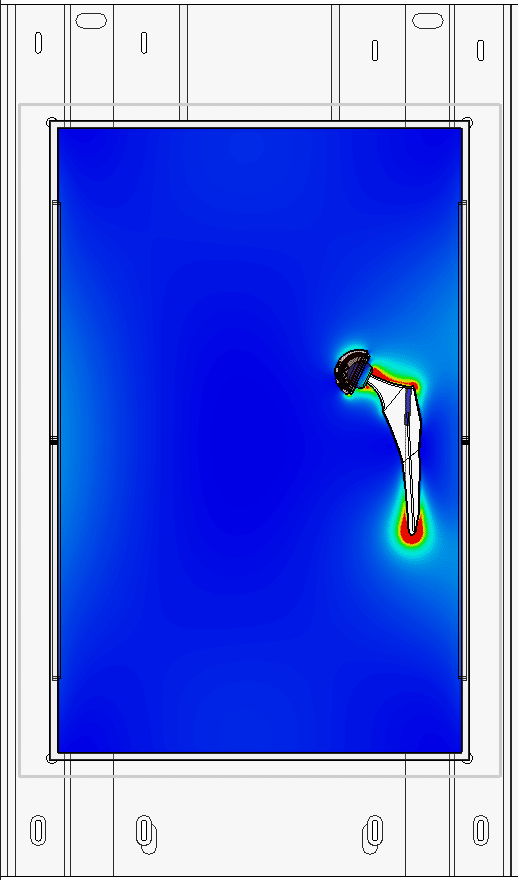
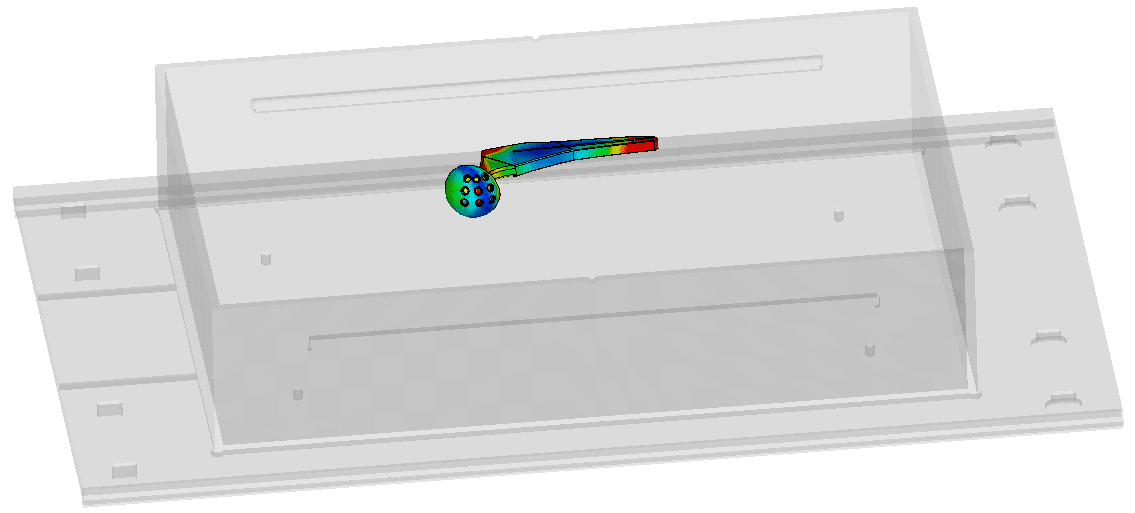
At Healtis, we offer numerical simulation services to help identify a worst-case configuration: the configuration that will heat up the most amongst the many possible clinical configurations.
These simulations can also be used to identify hot spots on the device, which will then be monitored when this worst-case configuration is tested in MRI.
Taking into account the bone surrounding orthopedic implants: an evolution of regulatory requirements (update December 2024)
The electrical properties of bone differ greatly from those of the gel defined in ASTM F2182. As a result, recent studies have shown that the worst cases determined in gel may turn out to be quite far from actual worst cases for devices mainly implanted in bone. Integrating the presence of bone tissue into a worst-case determination study therefore becomes essential for devices predominantly surrounded by bone.
We invite you to contact us to discuss this topic as we can guide you towards an optimal strategy for assessing the heating potentially induced by MRI radiofrequencies on your orthopedic implant.
In conclusion
For orthopedic systems (knee, hip, shoulder, trauma, etc..), the main difficulty in MRI risk assessment is generally the determination of worst-cases for the evaluation of radiofrequency-induced heating.
Simulations prove to be an effective and precise tool for optimizing this research. The number of configurations to be simulated remains to be defined: it will depend on the characteristics of the device and the rigor with which the manufacturer wishes to determine their worst-cases.
It should also be noted that the assessment of heating carried out under the conditions defined by ASTM F2182 may not be sufficient to correctly estimate the in vivo temperature expected for implanted medical devices, especially those implanted in bone. An additional step is often required to extrapolate test results and better estimate expected heating in humans.
Do you have any questions about this assessment? Contact us now at [email protected] !