Numerical Modeling: Simulations to assess the heating induced on your device by the radiofrequency fields of an MRI.
Medical devices may experience an increase in temperature as a result of the radiofrequencies (RF) present during an MRI exam. For implantable medical devices, partially implantable medical devices, or devices that may be in contact with a patient, this radiofrequency induced heating can present a risk and must be evaluated.
The radiofrequency induced heating depends on multiple factors: physical characteristics of the device, properties and settings of the MRI, positioning of the device, implantation conditions for an implantable medical device, etc.
The RF heating assessment can therefore be complex. The use of numerical simulations simplifies and consolidates these studies. As an expert in this field HEALTIS can accompany you:
- To determine worst-cases in ranges of passive medical devices with multiple configurations
- To determine the points of maximum heating on a medical device
- To translate the temperature increase measured in the ASTM gel phantom, to in-vivo tissue of interest (e.g. bone) and to in-vivo heating in humans.
- for specific studies requiring the modeling of radiofrequency heating.
Determination of the worst-case configuration
When a medical device comes in different configurations, it is difficult, if not impossible, to determine the configuration that will experience maximum radiofrequency induced heating through a theoretical or experimental approach.
Numerical modeling, sometimes coupled with statistical methods, provides solutions to enable the determination of a worst-case in a multi-feature device array. This device will generally be subjected to a physical heating test according to ASTM F2182.
We propose to realize this type of study for you.
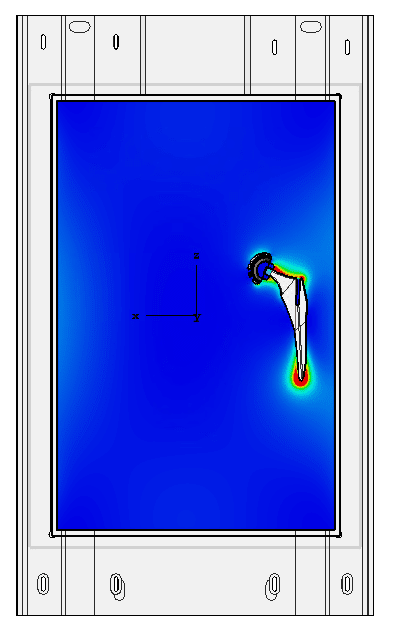
Determination of hot spots
The heating due to the radio frequencies is localized, and the measurement of this heating in MRI is done in an ad hoc way. On complex shapes it may be difficult to identify the points whose temperature should be monitored during the test. Numerical modeling makes it possible to predict these points of interest and thus to determine the location of the probes for measuring the temperature during the ASTM F2182 test.
Human Body Modeling: Translation of the radiofrequency induced heating test results to in vivo heating in humans
Radiofrequency induced heating is a function of, among other parameters, the local electric field, which may be different in the ASTM F2182 gel phantom than in human tissue. As a result, the temperature increase measured in the ASTM phantom may vary from the temperatures reached during the implanted patient MRI scan.
Using an FDA-approved computational modeling Medical Device Development Tool (MDDT), Healtis allows you to determine how this RF heating, measured in the ASTM F2182 gel phantom, translates into tissues of interest into in vivo heating in humans.
How do I select what device / device configuration for MRI safety testing ?
In the case where a device or a device component exists for which multiple configurations are possible, testing must be performed by considering a “worst-case” device configuration. This “worst-case” device configuration is the specific device configuration which is expected to experience the most severe results for the specific interaction of interest. The worst-case device configuration is often different depending on the tested interaction (e.g. the worst-case device sample for radio frequency induced heating and magnetically induced displacement force may not be the same device). Sample choices and testing conditions must be proven to be worst-case.
How do I determine the worst-case for the radiofrequency induced heating assessment ?
Determining the worst-case device is not a trivial selection. The largest device is not necessarily the worst-case for radiofrequency heating. Determining a worst-case for RF heating often requires a dedicated study involving either pre-tests or simulations.
Do I need to perform numerical modeling / simulations?
While the necessity of performing numerical modeling / simulations is very much determined on a case-by-case basis, for devices that are available in multiple configurations, simulations are strongly recommended to investigate which device is the worst-case.
There is also an increasing trend for authorities and notified bodies to request a “in-vivo scaling” or “human body modeling” in addition to ASTM F2182 testing. These types of simulations are meant to investigate how the phantom ASTM test results will translate to a human body. At the moment, these simulations are not a systematic request by notified bodies, but device manufacturers should be aware that these kinds of simulations may be asked of them.